SARS-CoV-2 IgM/IgG Rapid Test by Colloidal Gold Method
Recommended Applications
- As an auxiliary diagnosis of COVID-19
- Combined detection of IgM/IgG antibody and nucleic acid of SARS-CoV-2 can improve the detection rate
Advantages
-
Rapid
Instant results in 15 minutes -
Easy
Only 2 steps to operate -
Practical
1 Kit detect IgM and IgG antibodies -
Convenient
No need of any equipments or professionals -
Safe
Blood sample can minimize infection risk
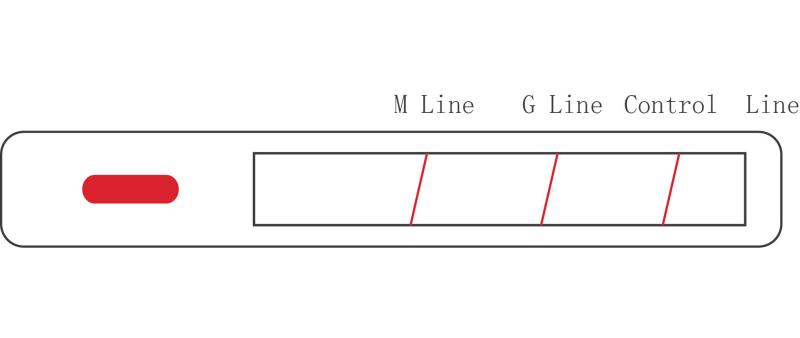
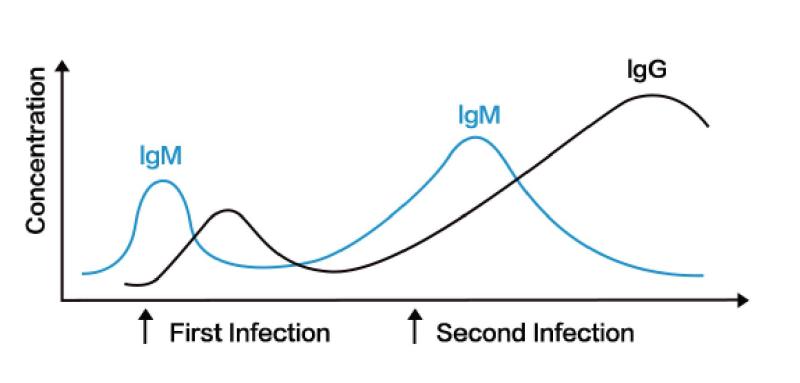
IgM can be detected within one week after infection with slight difference among indi- viduals. IgG appears about 14 days after infection and persists after production, and can be used as an indicator of previous infection. Monitoring both IgM and IgG makes the diagnosis more accurate.
Product Information
* Tests have been validated but FDA's independent review of this validation is pending.

About us
At Shanghai Biotecan Pharmaceuticals Co.,Ltd., our mission is to provide the best healthcare services and products through the most advanced technology. The company’s goal is to empower every medical professional and patient with systematic precision medicine solutions. Biotecan uses strict quality management control system and strictly follows China’s GLP & GMP standards. The company has over 60 clinical molecular diagnostic laboratories across China and two affiliated GMP facilities located in Shanghai and currently provides over 2000 different types of clinical testing services and 11 medical devices and diagnostic reagents.
Obtained certifications for SARS-CoV-2 detection were as follows:
- Novel Coronavirus 2019-nCoV Nucleic Acid Detection Kit (Real Time PCR) and SARS-CoV-2 IgM/IgG Rapid Test by Colloidal Gold Method developed by Biotecan have been both granted CE certification.
- Shanghai Biotecan Clinical laboratory successfully obtained NCCL EQA Certificate of SARS-CoV-2 detection.
- Shanghai Biotecan Clinical Laboratory successfully passed the SCCL verification of the insdetection capability for SARS-CoV-2.
- Shanghai Biotecan Clinical Laboratory is one of the institutions for SARS-CoV-2 nucleic acid testing designated by the Health Commission of Pudong new Area and Shanghai Municipal Health Commission.


